Carbohydrates are one of the most biologically active structures in nature. Structurally defined oligosaccharides play a key role in the communication between cells in higher organisms. Prominent examples are the blood group antigens which allow the differentiation between erythrocytes of different species and even between different individuals. Further recent discoveries in immunoncology regarding defined saccharides as targeting ligands for nanomedicine led to an increasing focus in our group towards the synthesis and biological application of complex saccharides.
Synthesis of clickable saccharides
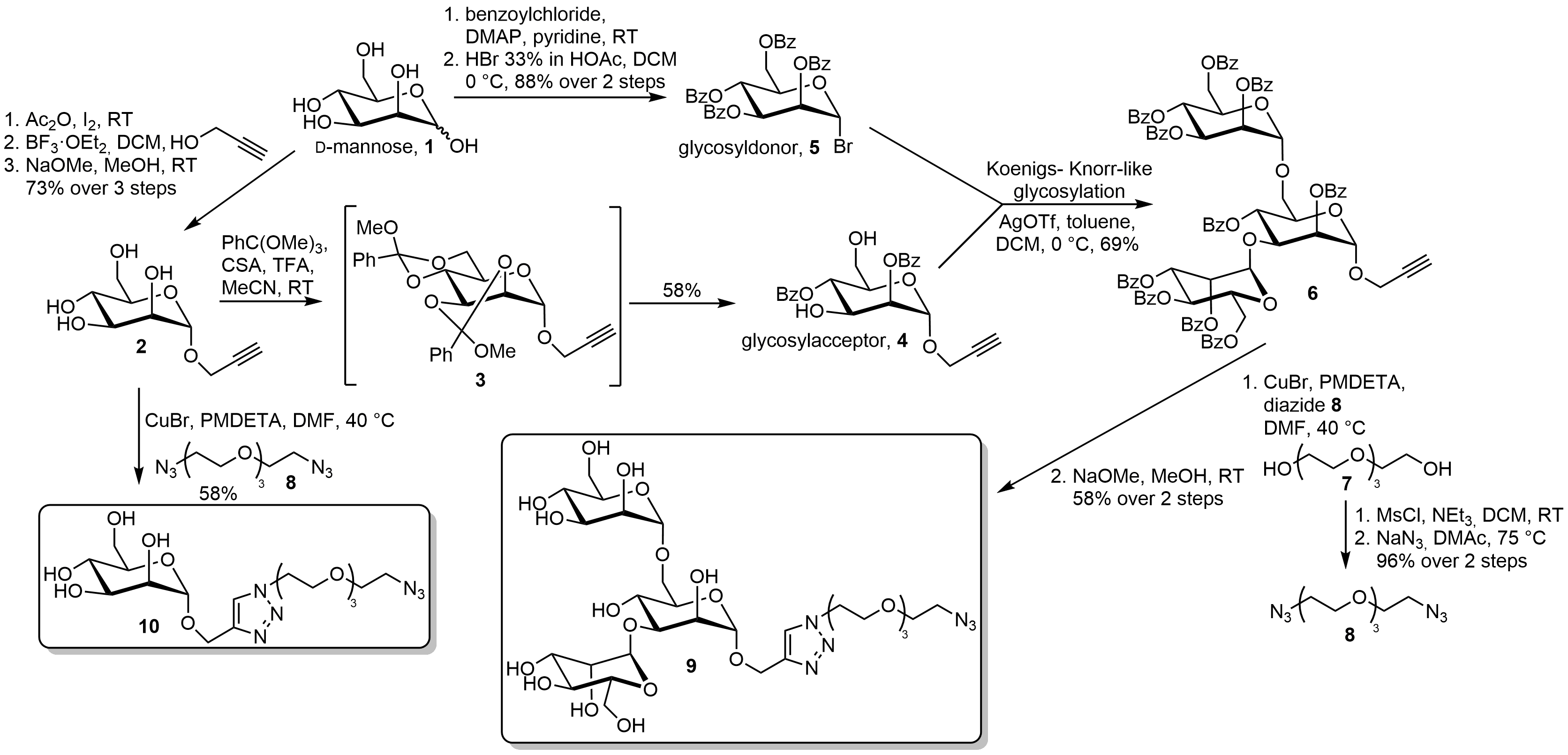
Nanomedicine is one of the key technologies in the 21st century. Besides the preparation and investigation of several nano-carriers and protein repellent surfaces the development of specific cell targeting is very important. Mannose and Polymannose-glycans are well known ligands of the DC-SIGN and Langerin receptors. These can be found on the surface of dendritic cells and belong to the C-type lectins. The C-type lectins are carbohydrate binding proteins and play key roles, e. g. in cell-cell-adhesion or the immune system. Our group investigated and developed a short synthesis for clickable carbohydrate-ligands for application onto nano-carriers.
Sweet (Hetero)aromatics
Mono- and diglycosylated aromatics as well as heteroaromatics may serve as metabolically stable mimetics of di- and trisaccharides. In our group, we investigate methods for their preparation from monosaccharidic building blocks e.g. by direct C-glycosylation, alkyne hydroamination or Larock cyclization.

Selected Publications:
Bioconjugation of small molecules to RNA impedes its recognition by Toll-like receptor 7 (TLR7)
I. Hellmuth, I. Freund, J. Schlöder, S. Seidu-Larry, K. Thüring, K. Slama, J. Langhanki, S. Kaloyanova, T. Eigenbrod, M. Krumb, S. Röhm, K. Peneva, T. Opatz, H. Jonuleit, A. H. Dalpke, M. Helm, Front. Immunol. 2017, 8, 312.
Carbohydrate-Based Nanocarriers Exhibiting Specific Cell Targeting with Minimum Influence from the Protein Corona
B. Kang, P. Okwieka, S. Schöttler, S. Winzen, J. Langhanki, K. Mohr, T. Opatz, V. Mailänder, K. Landfester, F. R. Wurm, Angew. Chem. 2015, 127, 7544–7548.
Chemoenzymatic Synthesis of Functional Sialyl Lewisx-Mimetics with a Heteroaromatic Core
C. Schlemmer, C. Wiebe, D. Ferenc, D. Kowalczyk, S. Wedepohl, P. Ziegelmüller, J. Dernedde, T. Opatz, Chem. Asian J. 2014, 9, 2119–2125.
Synthesis of 1,3- and 2,3- Diglycosylated Indoles as Potential Trisaccharide Mimetics
C. Wiebe, S. Fusté de la Sotilla, T. Opatz, Synthesis 2012, 44, 1385–1397.